Background context:
Clinical trials rely on detailed protocols—guidelines that dictate how a trial is run. At Slope, our users depend on accurate, easy-to-manage protocol configurations to ensure quality and regulatory compliance. This is the foundation of or product, and every aspect of the Clinical trial management in Slope connects to the protocol.
However, managing protocols in our platform was slow, error-prone, and misaligned with how trials are structured in the real world. With upcoming partnerships with global pharma companies, we needed to rethink our approach to scale effectively.
The Problems: Our legacy protocol management system couldn't support:
Full protocol lifecycle
External user acceptance testing (UAT)
Easy versioning (amendments)
Data accuracy and control at scale
This limited our ability to grow and required heavy manual intervention.
Goal: Redesign protocol management so it:
Scales without manual work
Reflects real-world clinical trial workflows
Reduces risk of data and configuration errors
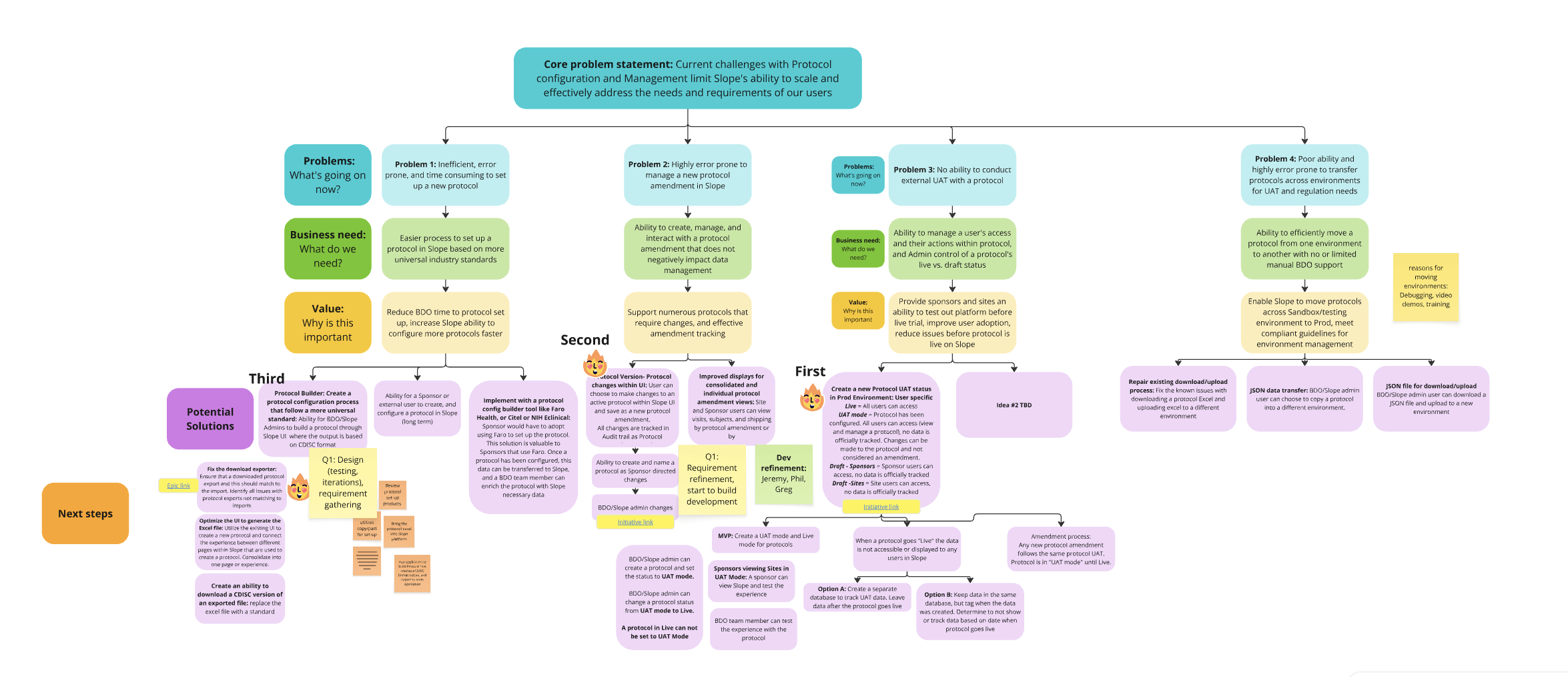
Opportunity Solution tree mapping
Led brainstorming sessions and workshops to define the solution
Solution:
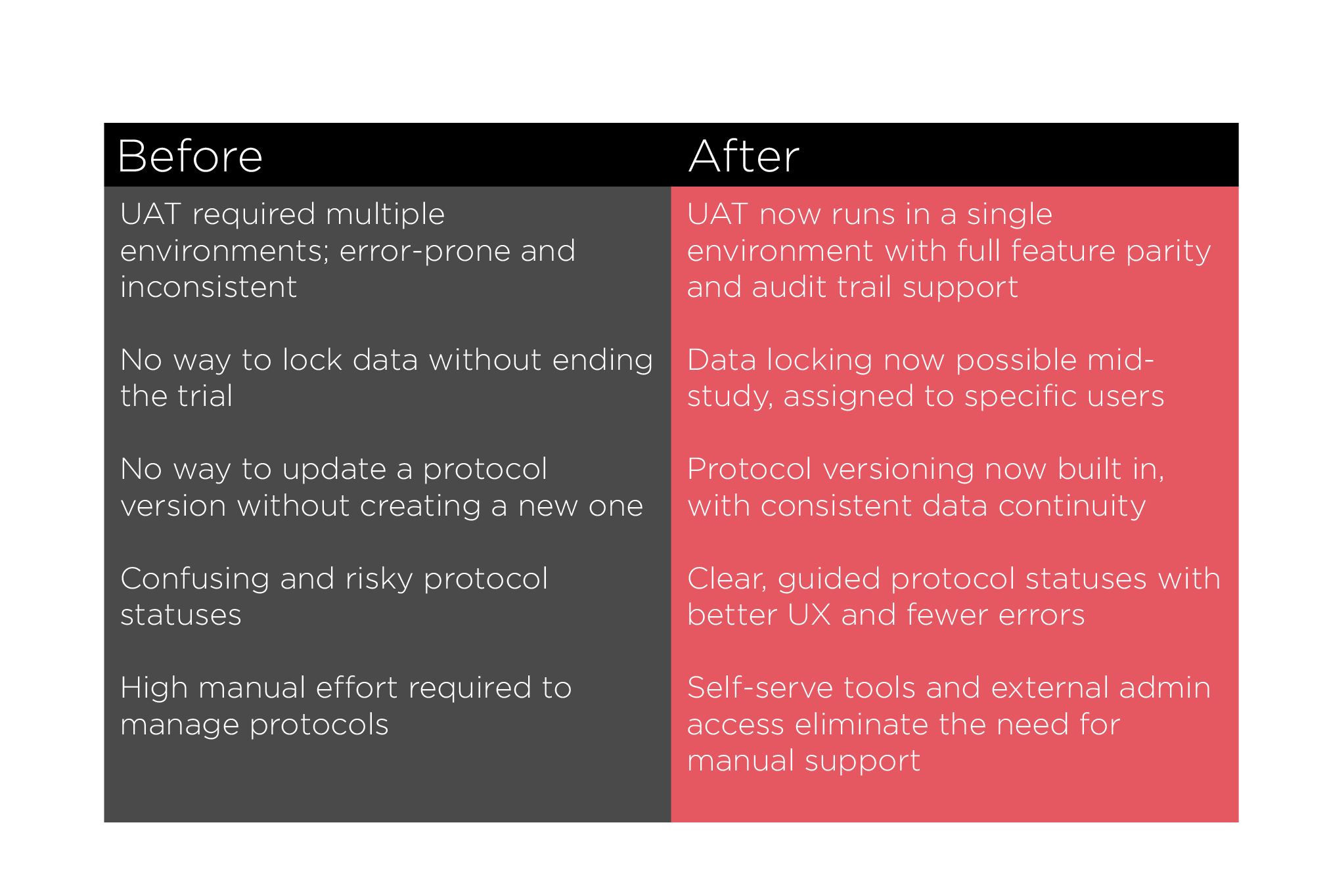
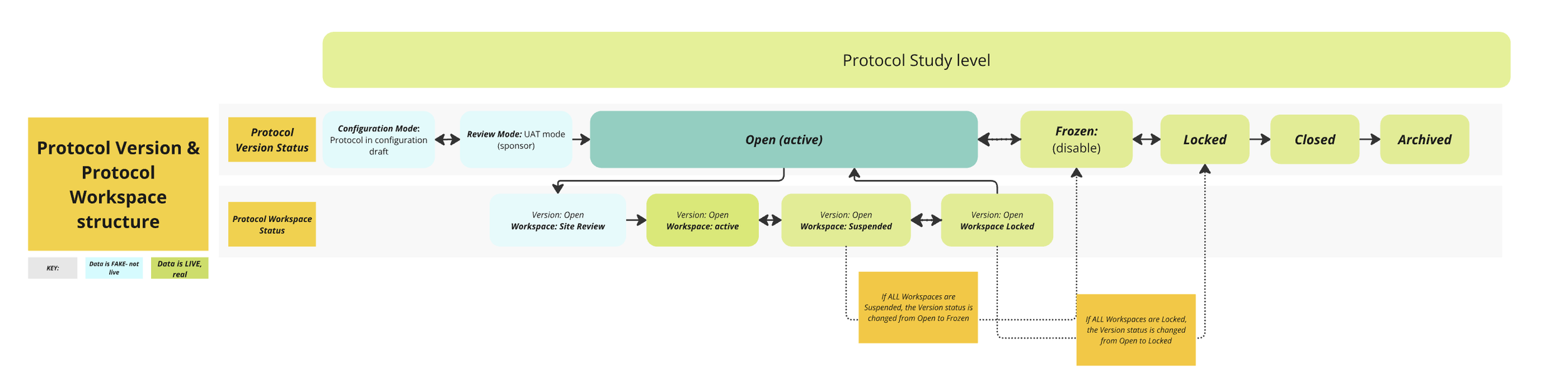
We built a new protocol management structure in our platform that mirrors how clinical trials actually operate—streamlining processes, reducing risk, and improving user experience. We introduced a new protocol management structure that included:
Lifecycle-based protocol statuses (e.g., Draft, UAT, Active, Locked)
Support for versioning and protocol amendments
Better data integrity and tracking across the trial lifespan
This foundation created a scalable, user-friendly experience that aligned with industry standards and unlocked Slope’s ability to support larger, global trials.
How did we get there?
Identified core problems and aligned on business value
Validated ideas through stakeholder interviews and small-scale testing
Determined that short-term fixes (like manual file uploads) wouldn't scale
Designed a foundational solution that supports long-term growth and enterprise needs
Product methods used:
Opportunity Solution Mapping
Requirements Gathering & Prioritization
User Flow Design
Competitive & Industry Analysis
Stakeholder Alignment
NEW: more organized, industry-aligned Platform protocol and versioning structure
Lessons Learned:
1. Quick fixes can cost more than foundational change.
Early on, there was pressure to “just patch what we have” rather than rebuild. But after evaluating both paths, it became clear: temporary fixes were more expensive long-term and didn’t solve our scalability or data integrity issues. Rebuilding the protocol structure from the ground up was the most strategic and cost-effective choice. Once aligned, we launched an MVP with a structured rollout to minimize disruption.
2. Identify root causes, not just symptoms.
We collected 50+ user complaints and requests—but many were symptoms of deeper issues. By zooming out, we uncovered recurring themes around lifecycle gaps, lack of version control, and limited UAT capabilities. Solving these root problems avoided wasteful investments in isolated features that wouldn't scale—or worse, introduced new risks.